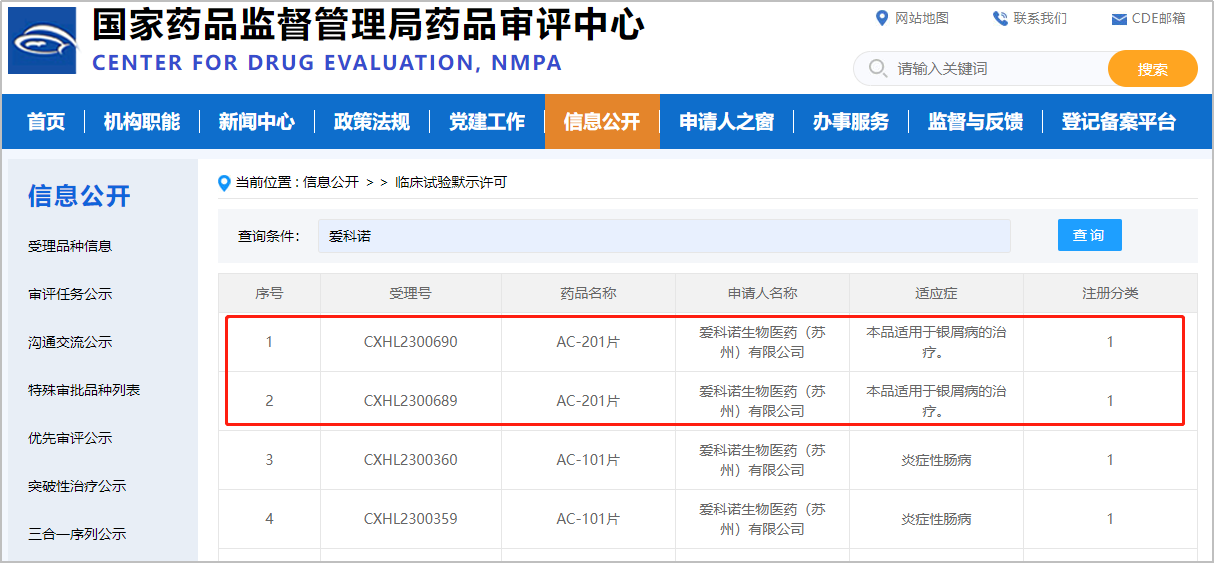
Suzhou China, September 8, 2023 —— Accro Bioscience (Suzhou) Limited (Accropeutics) announced today that its oral small-molecule TYK2/JAK1 inhibitor AC-201 has been approved by the National Medical Products Administration (NMPA) to launch clinical phase 1 trial in China. AC-201 was approved for clinical trials by HREC in Australia in May 2023, and the Phase 1 clinical trial in Australia is currently underway.
This is the fifth clinical approval obtained by Accropeutics this year, and the seventh since its very founding in 2017.
AC-201 is a novel oral small-molecule inhibitor of TYK2/JAK1 with high activity, selectivity, and safety window. The preclinical studies have shown that AC-201 can effectively bind to the pseudo kinase domain (JH2) of TYK2/JAK1, stabilizing the self-inhibitory conformation of the pseudo kinase domain on the kinase domain, thereby inhibiting the function of TYK2/JAK1 kinase. It demonstrated significant efficacy in multiple animal models including psoriasis and is intended for the treatment of psoriasis and other inflammatory and autoimmune diseases.
About Accropeutics
Accropeutics is a clinical-stage biotech company with a core focus on molecular mechanisms of regulated cell death and related pathogenesis in human diseases. The Company has developed a robust portfolio with innovative compounds in various stages spanning from lead optimization to clinical trials. The company's RIPK1 inhibitor AC-003 has completed the Phase 1 clinical trial between China and the United States in August 2023 and achieved positive results, and is ready to dose patients for GVHD. The company’s RIPK2 inhibitor AC-101 was approved by HREC in March 2023 and is about to complete its Phase 1 clinical trial. AC-201, a selective TYK2/JAK1 inhibitor with huge potential for treating inflammatory and autoimmune diseases, was approved by HREC and NMPA in May 2023 and September 2023, a phase 1 clinical trial is underway in Australia, and is about to launch clinical trials in China. The company has multiple compounds in the PCC and preclinical research and development stages. Accropeutics owns global rights of all its assets with more than 10 patents issued in China, Japan, Korea, US and EU.